(PharmaNewsWire.Com, June 25, 2025 ) In Vitro Diagnostics (IVD) Market: Accelerating Growth Through Technological Advancements and Precision Medicine
The In Vitro Diagnostics (IVD) market size s undergoing rapid transformation, powered by innovations in molecular biology, automation, and personalized medicine. IVD refers to tests performed on biological samples such as blood, urine, or tissues to diagnose diseases or monitor overall health. This sector plays a critical role in disease detection, therapy monitoring, and risk assessment—making it one of the pillars of modern healthcare. It is a dynamic and fast-growing sector, essential to the evolution of precision medicine and modern healthcare delivery. For stakeholders ranging from investors to diagnostics developers, this market presents a wide spectrum of innovation-driven growth opportunities.
Market Overview
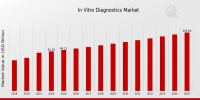
This growth is fueled by an aging population, rising incidence of chronic diseases, and increased demand for early and accurate diagnostics.As per MRFR analysis, the In Vitro Diagnostics Market Size was estimated at 78.36 (USD Billion) in 2023.The In Vitro Diagnostics Market Industry is expected to grow from 81.19(USD Billion) in 2024 to 120 (USD Billion) by 2035. The In Vitro Diagnostics Market CAGR (growth rate) is expected to be around 3.61% during the forecast period (2025 – 2035).
Request To Free Sample of This Strategic Report: https://www.marketresearchfuture.com/sample_request/1165
The COVID-19 pandemic significantly accelerated IVD usage, creating a long-term impact on testing practices and reinforcing the importance of rapid diagnostic capabilities. Post-pandemic, the market is evolving toward home-based diagnostics and precision testing tailored to individual needs.
Market Growth Drivers
Rising Prevalence of Chronic and Infectious Diseases
Increasing global rates of diabetes, cancer, cardiovascular diseases, and infectious illnesses are amplifying the demand for timely diagnostic tools.
Technological Advancements in Testing Methods
Innovations such as next-generation sequencing (NGS), real-time PCR, point-of-care diagnostics, and AI-integrated lab systems are boosting diagnostic speed, accuracy, and convenience.
Shift Toward Personalized Medicine
Tailored treatments based on genetic profiles require highly sensitive and specific diagnostic platforms—driving demand for molecular diagnostics and companion diagnostics.
Home and Remote Testing Trends
The rise in telehealth has supported the adoption of home-use kits for glucose monitoring, fertility tracking, and infectious disease detection.
Supportive Government Policies and Investments
Public-private collaborations, grants, and favorable reimbursement policies have helped promote IVD adoption in both developed and emerging economies.
Key Companies in the In Vitro Diagnostics Market Include
Abbott Laboratories
Hologic
Thermo Fisher Scientific
QIAGEN
Johnson and Johnson
Danaher Corporation
Sysmex Corporation
Roche Diagnostics
Archers Health
Becton Dickinson
PerkinElmer
Cepheid
Ortho Clinical Diagnostics
Siemens Healthineers
bioMérieux
Market Dynamics
Opportunities:
Expansion in emerging markets due to improving healthcare infrastructure
Growing demand for liquid biopsy and non-invasive diagnostics
AI-driven automation in laboratory testing
Rising health consciousness and preventive healthcare measures
Challenges:
High cost of advanced IVD instruments and reagents
Regulatory hurdles delaying product approvals
Data integration issues between diagnostic platforms and healthcare systems
Trends:
Increased demand for rapid diagnostic tests (RDTs)
Integration of big data and analytics for better diagnostic insights
Emergence of wearable diagnostics and mobile apps
Buy Now Premium Research Report : https://www.marketresearchfuture.com/checkout?currency=one_user-USD&report_id=1165
In Vitro Diagnostics Market Segmentation Insights
In Vitro Diagnostics Market Test Type Outlook
Clinical Chemistry
Microbiology
Immunology
Molecular Diagnostics
Hematology
In Vitro Diagnostics Market Product Outlook
Reagents
Instruments
Software
Quality Control Products
Consumables
In Vitro Diagnostics Market End User Outlook
Hospitals
Diagnostic Laboratories
Academic and Research Institutions
Home Care Settings
In Vitro Diagnostics Market Application Outlook
Infectious Diseases
Diabetes
Oncology
Cardiovascular Diseases
Genetic Testing
In Vitro Diagnostics Market Regional Outlook
North America
Europe
South America
Asia Pacific
Middle East and Africa
Industry Developments
The In Vitro Diagnostics (IVD) market is witnessing dynamic industry developments, particularly through strategic mergers and acquisitions, product innovations, and regulatory advancements. Leading companies such as Roche, Thermo Fisher Scientific, and Danaher are actively acquiring niche firms to broaden their product portfolios, especially in high-growth areas like molecular diagnostics and companion diagnostics. Simultaneously, the market is experiencing a surge in the launch of innovative diagnostic tools, including multiplex PCR kits and rapid point-of-care antigen tests, catering to the growing demand for quick and accurate testing solutions. Regulatory bodies such as the FDA and European Commission are also playing a pivotal role by expediting approvals for diagnostic products that address critical and unmet medical needs, further accelerating market expansion and encouraging innovation across the industry.
Browse In-depth Market Research Report (128 Pages, Charts, Tables, Figures) on In Vitro Diagnostics (IVD) Market:
https://www.marketresearchfuture.com/reports/in-vitro-diagnostics-market-1165
Key Stakeholders
The In Vitro Diagnostics (IVD) market is shaped by a diverse set of key stakeholders, each playing a critical role in its growth and innovation. Diagnostic companies such as Roche, Abbott, Siemens Healthineers, Thermo Fisher Scientific, Bio-Rad Laboratories, and Danaher Corporation lead the market with robust portfolios and continuous advancements in diagnostic technologies. Hospitals and laboratories serve as the primary users of these sophisticated IVD systems, employing them for both routine testing and complex disease diagnostics. Research institutions contribute significantly by pioneering new biomarker discoveries and advancing genetic testing methodologies. Governments and regulatory bodies influence the market through strategic funding initiatives, policy frameworks, and the approval of diagnostic products, ensuring safety and efficacy standards. Additionally, investors are vital stakeholders, fueling innovation and expansion by backing startups and emerging technologies that promise to revolutionize diagnostics and improve patient outcomes.
Reasons to Buy the Report
Understand market trends and growth projections up to 2035 across major geographies and segments
Analyze key players’ strategies, partnerships, and product innovations
Evaluate opportunities in emerging markets, especially in Asia-Pacific and Latin America
Benchmark technology adoption, pricing, and reimbursement dynamics
Identify growth drivers and risks to support informed investment and business decisions
Related Report
Advanced Baby Monitors Market: https://www.marketresearchfuture.com/reports/advanced-baby-monitors-market-6525
Thyroid Disorder Market: https://www.marketresearchfuture.com/reports/thyroid-disorder-market-2747
Suture Needle Market: https://www.marketresearchfuture.com/reports/suture-needles-market-552
Ultra-Low Temperature Freezers Market: https://www.marketresearchfuture.com/reports/ultra-low-temperature-freezer-market-1813
PEGylated Drugs Market: https://www.marketresearchfuture.com/reports/pegylated-drugs-market-8436
Market Research Future
Market Research Future
+1 (646) 845 9349
sales@marketresearchfuture.com
Source: EmailWire.Com
Source link